Blog
The Role of Chest Imaging in Patient Management during the COVID-19 Pandemic: A Multinational Consensus Statement from the Fleischner Society
With more than 900,000 confirmed cases worldwide and nearly 50,000 deaths during the first three months of 2020, the COVID-19 pandemic has emerged as an unprecedented healthcare crisis. The spread of COVID-19 has been heterogeneous, resulting in some regions having sporadic transmission and relatively few hospitalized patients with COVID-19 and others having community transmission that has led to overwhelming numbers of severe cases. For these regions, healthcare delivery has been disrupted and compromised by critical resource constraints in diagnostic testing, hospital beds, ventilators, and healthcare workers who have fallen ill to the virus exacerbated by shortages of personal protective equipment. While mild cases mimic common upper respiratory viral infections, respiratory dysfunction becomes the principal source of morbidity and mortality as the disease advances. Thoracic imaging with chest radiography (CXR) and computed tomography (CT) are key tools for pulmonary disease diagnosis and management, but their role in the management of COVID-19 has not been considered within the multivariable context of the severity of respiratory disease, pre-test probability, risk factors for disease progression, and critical resource constraints. To address this deficit, a multidisciplinary panel comprised principally of radiologists and pulmonologists from 10 countries with experience managing COVID-19 patients across a spectrum of healthcare environments evaluated the utility of imaging within three scenarios representing varying risk factors, community conditions, and resource constraints. Fourteen key questions, corresponding to 11 decision points within the three scenarios and three additional clinical situations, were rated by the panel based upon the anticipated value of the information that thoracic imaging would be expected to provide. The results were aggregated, resulting in five main and three additional recommendations intended to guide medical practitioners in the use of CXR and CT in the management of COVID-19.
Essentials
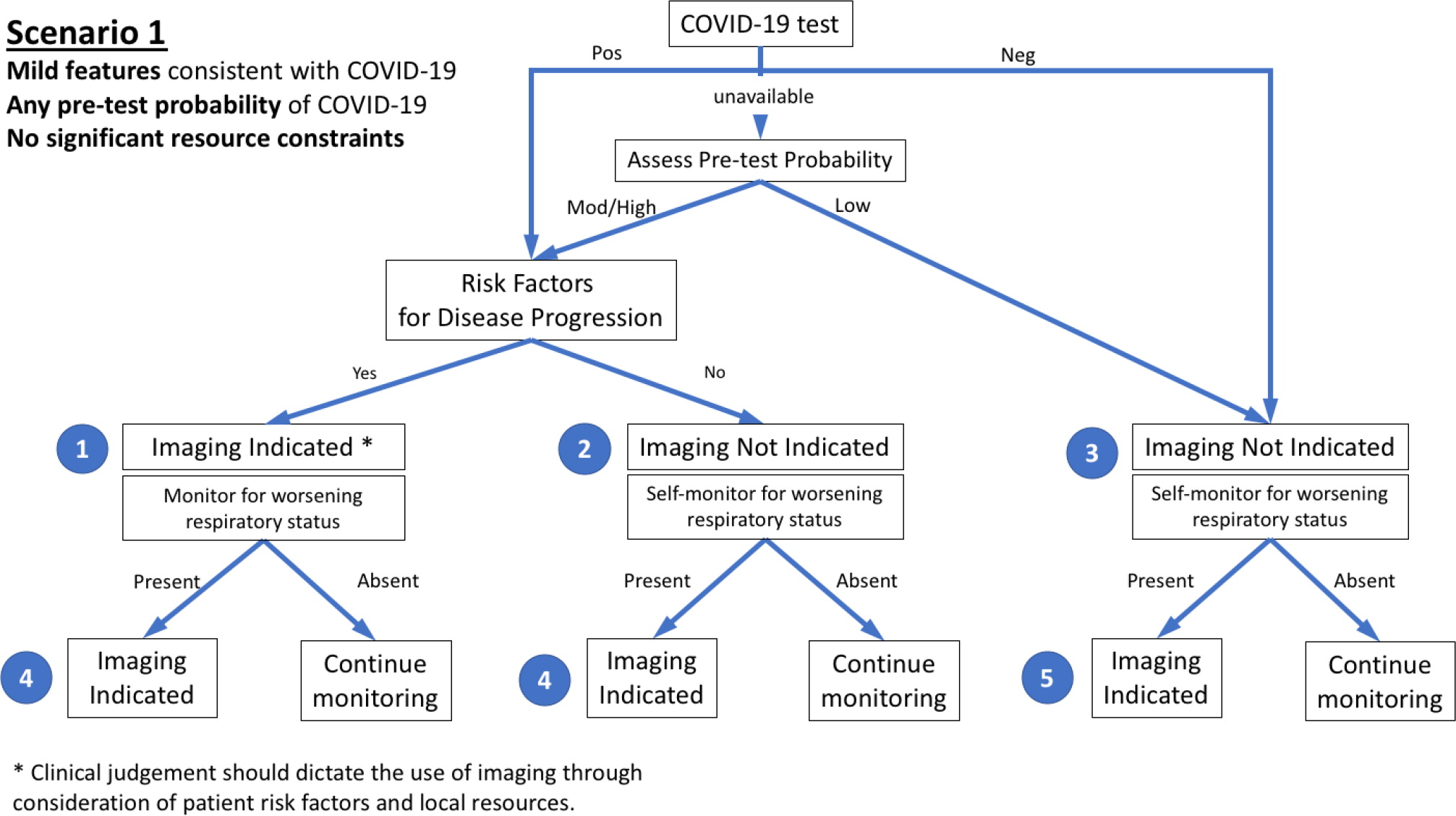
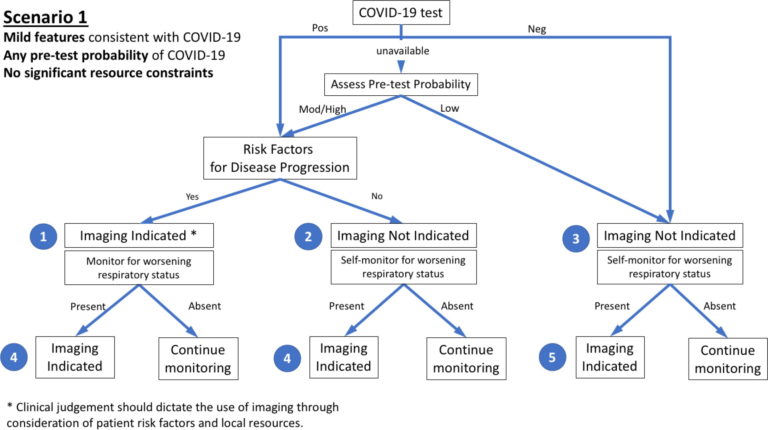
■ Imaging is not indicated in patients with suspected COVID-19 and mild clinical features unless they are at risk for disease progression.
■ Imaging is indicated in a patient with COVID-19 and worsening respiratory status.
■ In a resource-constrained environment, imaging is indicated for medical triage of patients with suspected COVID-19 who present with moderate-severe clinical features and a high pre-test probability of disease.
Introduction
On March 11, 2020 the World Health Organization (WHO) officially characterized the rapid global spread of coronavirus disease 2019 (COVID-19) as a pandemic and called for urgent international action in four key areas: to prepare and be ready; detect, protect, and treat; reduce transmission; and innovate and learn (1). At the time of writing (April 1, 2020), there are over 900,000 confirmed COVID-19 cases and nearly 50,000 deaths in 205 countries around the world, with the majority of cases concentrated in 4 countries: United States, Italy, Spain, and China (2, 3). With sustained community transmission now established in multiple countries on multiple continents, the WHO public health goal has changed from containment to mitigation of the pandemic’s impact. Consequently, strategies are now focused on efforts to reduce the incidence, morbidity, and mortality of COVID-19 by breaking the chain of human transmission through social distancing and imposed quarantine.
Diagnostic Testing
Early detection and containment of infection caused by the novel coronavirus SARS-CoV2 has been hindered by the need to develop, mass produce, and widely disseminate the required molecular diagnostic test, a real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay. Early reports of test performance in the Wuhan outbreak showed variable sensitivities ranging from 37% to 71% (4, 5). While laboratory-based performance evaluations of RT-PCR test show high analytical sensitivity and near-perfect specificity with no misidentification of other coronaviruses or common respiratory pathogens, test sensitivity in clinical practice may be adversely affected by a number of variables including: adequacy of specimen, specimen type, specimen handling, and stage of infection when the specimen is acquired (CDC guidelines for in-vitro diagnostics) (6, 7). False negative RT-PCR tests have been reported in patients with CT findings of COVID-19 who were eventually tested positive with serial sampling (8). Limited testing capacity due to insufficient specimen collection kits, lab test supplies, and testing equipment precluded early widespread testing and is believed to have contributed to rapid and unchecked transmission of infection within communities by undetected individuals with milder, limited, or no symptoms (9, 10). For example, CT screening of 82 asymptomatic individuals with confirmed COVID-19 from the cruise ship “Diamond Princess” showed findings of pneumonia in 54% (11).
To read the full article, please follow below link:
Recent Posts
Archives
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- April 2024
- February 2024
- January 2024
- April 2022
- November 2021
- August 2021
- May 2021
- April 2021
- March 2021
- February 2021
- December 2020
- November 2020
- October 2020
- July 2020
- April 2020
- December 2019
- October 2019
- August 2019
- July 2019
- June 2019
- April 2019